According to Wikipedia, "Nuclear power plants have a carbon footprint comparable to that of renewable energy such as solar farms and wind farms, and much lower than fossil fuels such as natural gas and coal."
In a news article in 2024, a minister announced that "Singapore has plans to build a pool of about 100 nuclear energy experts" though "no decision has been made on the deployment of nuclear energy".
Rutherford α-particle scattering
Around the start of the 20th century (again, yes, lots of new physics were discovered around that time), Ernst Rutherford was a scientist in Manchester, England. One day in 1909, he asked his friends Hans Geiger and Ernest Marsden to do an experiment. He asked them to shoot some alpha particles at a thin, gold foil.
Alpha particles are part of the radiation from radioactive materials. At the time, alpha particles were probably to be some kind of very tiny particles travelling at super high speed.
To appreciate what happened, we need some background. That was a time when scientists had a vague idea that matter was made up of atoms, but had no idea what the inside an atom look like. The simple thinking was that maybe atoms were solid balls. See my earlier article on ideal gas.
Geiger and Marsden carried out the experiment. They found to everyone's surprise that most of the alpha particles went through the gold foil as if it was not there. Some scattered in other directions, and a small amount bounced right back!
Why were they surprised? At that time, people thought an atom was like a pice of pudding, and electrons were like tiny bits of plum stuck inside the pudding.
"The funny thing is, a plum pudding does not contain any plum! This goes back to the Victorian practice of substituting dried plums with other dried fruits, such as raisins. Dried plums or prunes were so popular that any goods which contained dried fruits were referred to 'plum cakes' or 'plum puddings'."

Rutherford thought the atom was like a ... plum pudding. And the alpha particle like bullets. So what would you expect if you shoot a bullet at a pudding? The bullet would just go straight through as if the pudding is not there!
I have a question though - if they were so sure about this, why did they bother to do this experiment? Maybe Rutherford just wanted to check, Scientists always wanted to be sure. And this is a new experiment, so I guess it was worth doing. There is always the chance to find some a bit different, which they could published and get some credit.
Instead of just getting some credit, What they found changed the world.
They found that although most of the alpha particles when straight through the gold foil, a small fraction bounced right back!
An ordinary student would probably dismiss this as experimental error or not important. But Rutherford was no ordinary student. He sensed that they have discovered something big.
Rutherford was quoted as saying: "It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you."
The gold foil ... was a very expensive piece of tissue paper.
The rest was history. The discovery provided ideas that led to more work and many discoveries in nuclear physics. Here is a Rutherford model picture of an atom.

Nucleon number (mass number) and proton number
Today, we learn that most of the mass of an atom is in a small volume at the centre - called the nucleus. This nucleus contains two types of particles - protons and neutrons. The rest of the volume of the atom are most empty space with a number of relative light electrons.
The proton and the neutron have nearly equal mass. The electron is much lighter, nearly 2000 times lighter that a proton.
The protons and neutrons in a an atom are collectively called "nucleons". The total number of nucleons in a atom is called "nucleon number", or more commonly the "mass number".
Tne number of protons in an atom is called the "atomic number". It is used to arrange the order of elements on a periodic table, a table that list all the 100 plus elements that exist.
Before we get too abstract, lets take a look at what all these have to do with our life. As they say, a picture is worth 1000 words. And so I found my favourite periodic table.
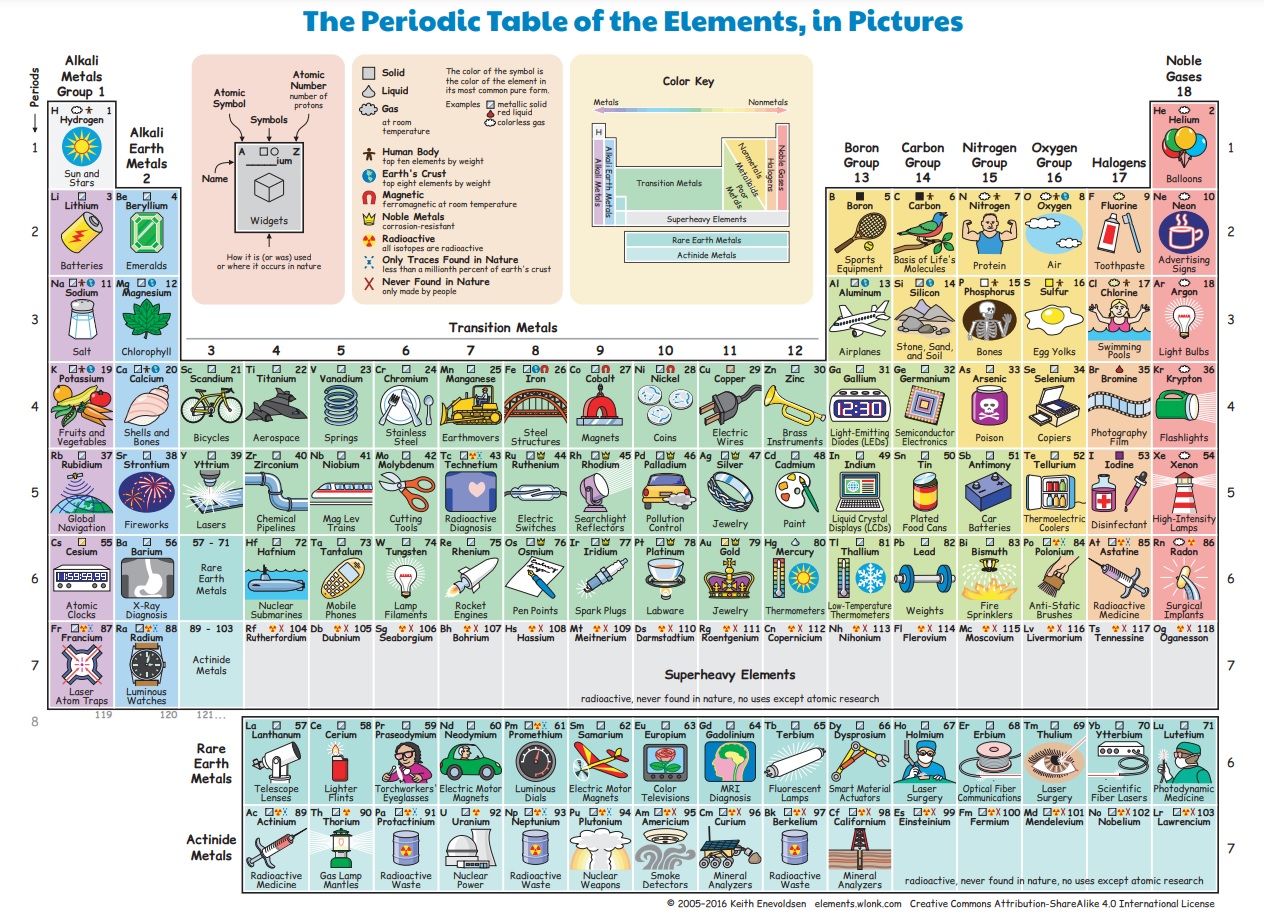
A periodic table is basically a list of all the elements in the world, maybe even the universe. But what is an element? To understand this, we need a bit of chemistry.
Isotopic forms of elements
As an example, lets look at water, the clear, flowing liquid that give us life. About 60% of our body is water. Today most people know that water is H2O. Many of would also know that H is hydrogen and O is oxygen.
But maybe not everyone knows what the hydrogen or oxygen atom is made up of. A hydrogen atom consists of a proton at the centre, and an electron around it. An oxygen atom has 8 protons and 8 neutrons at the centre.
It is the number of protons that determine what element it is, because te protons are positively charged and affects the atom's electrons - which affects how the chemical reaction with other atoms.
For example, it is possible for a hydrogen atom to have an additional neutron. This would still react chemically like hydrogen, like burning in oxygen. The atom would just be heavier. But if the hydrogen has an additional proton, it would not be hydrogen any more - it would become a helium atom, which is inert and cannot burn like hydrogen.
The hydrogen atom with an additional neutron can still be called hydrogen, though it is given the name deuterium. It can still burn in oxygen to give ... water. Yes, it still looks and taste like water, but its density is a bit higher. So this water is normally called heavy water.
According to Wikipedia, small amounts are safe for living things, but large amounts of heavy water would affect their health.
Nuclear Reactions
If you have learnt some chemistry, you would know for example that hydrogen can burn in oxygen to give water:
Atoms in the original hydrogen and oxygen molecules end up getting rearranged into water molecules. The main action takes place among the electrons. In this chemical reaction, the nucleus of each atom remain unchanged. So the hydrogen is still hydrogen, the oxygen still oxygen.
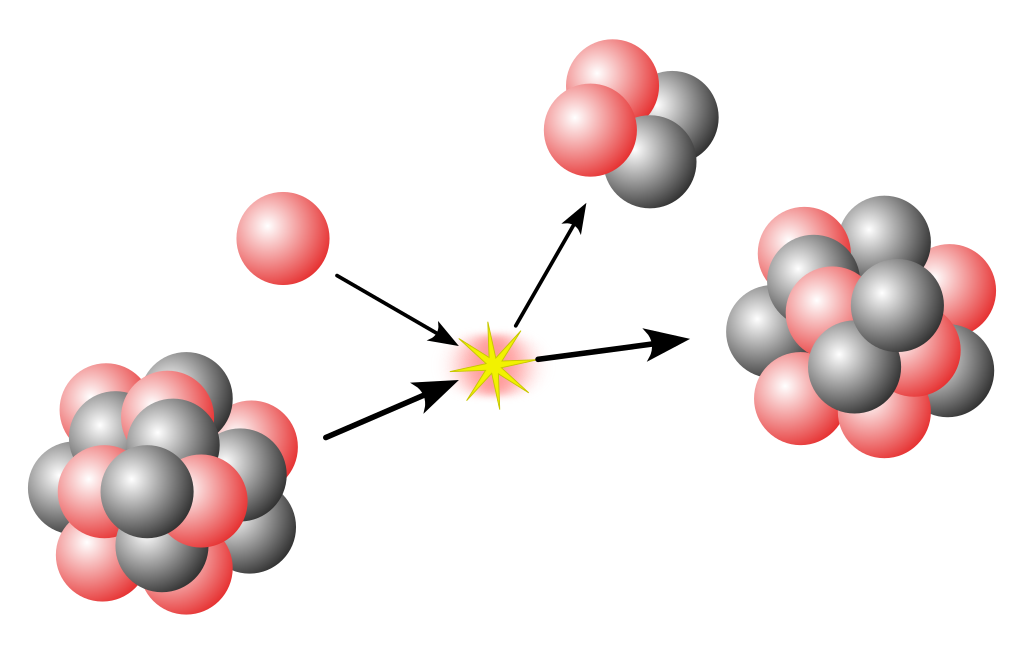
Now lets look at a nuclear reaction. This is a reaction between bare nuclei, usually with electrons stripped off. In this example, a nitrogen nucleus hits a helium nucleus.
And we end up getting an oxygen nucleus and a hydrogen nucleus. The nitrogen and helium we started with do not exist anymore!
So an element has changed into a different element. This is called transmutation. Many hundred years ago, alchemists have tried changing lead to gold. They failed. Today, we know that this would require changing the number of protons and neutrons.
Conservation of Nucleon number, Charge and Mass-Energy
The equation for the nuclear reaction above looks a bit like the equation of a chemical reaction, e.g.
Note that in a chemical reaction, the number of atoms of each element remain the same after the action.
But unlike hydrogen and oxygen reacting to give water, nuclear reactions involve only the nuclei. For example, the Nitrogen and Helium above probably had all their electrons removed, so that the nuclei smash directly into each other.
The following animation is an artist impression of a hydrogen nucleus smashing into a helium nucleus.
First
Then
which decomposes into alpha particles
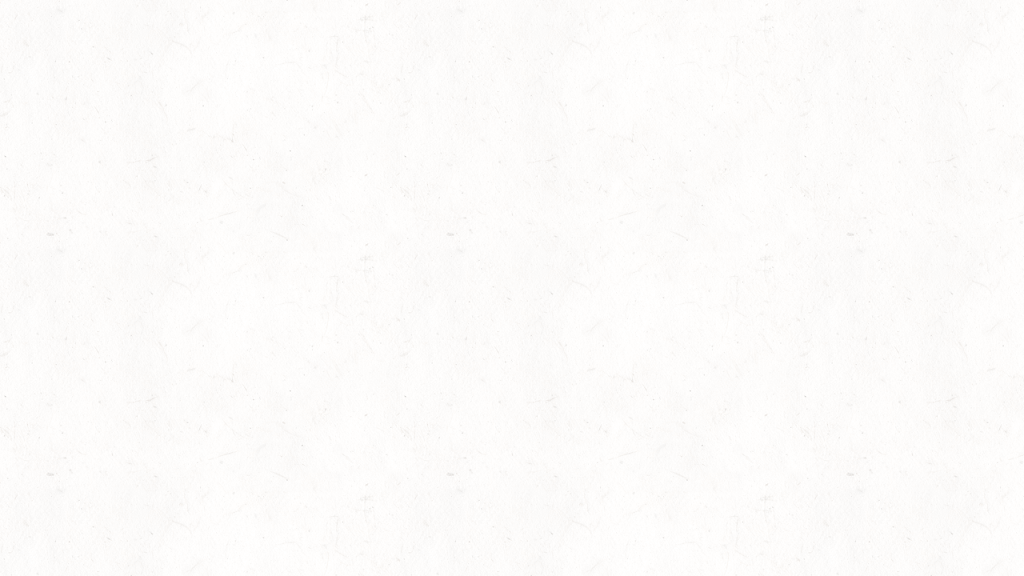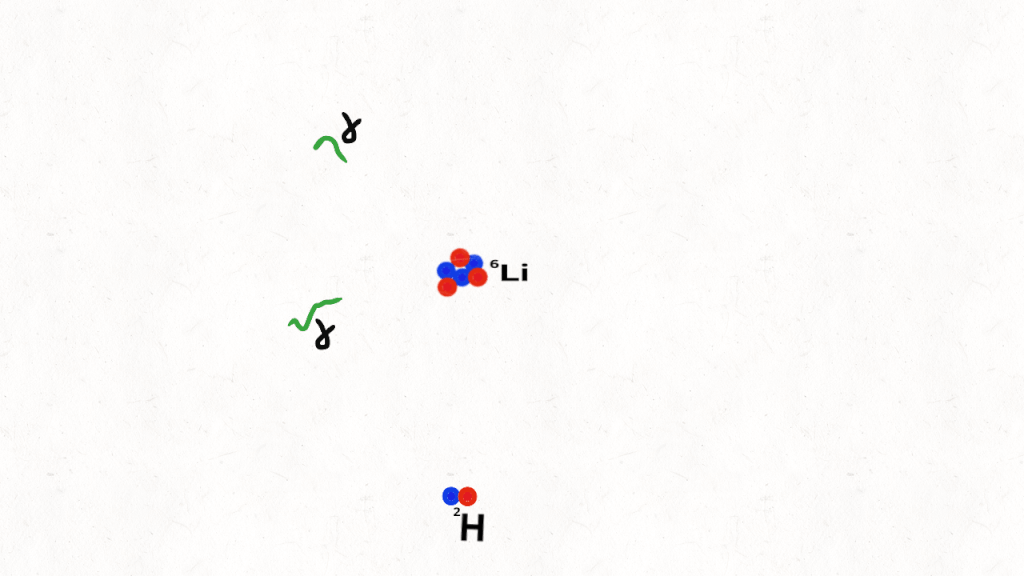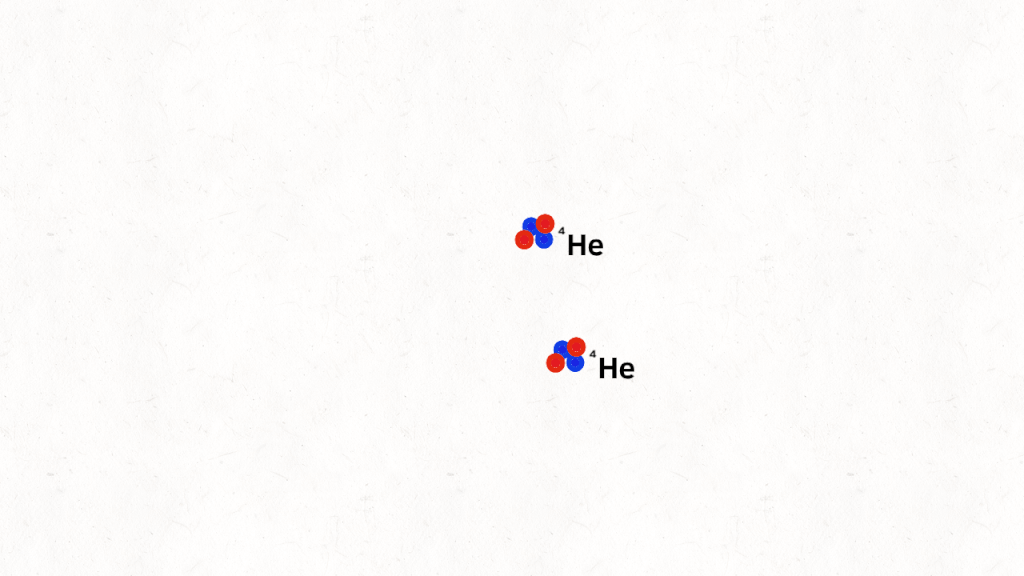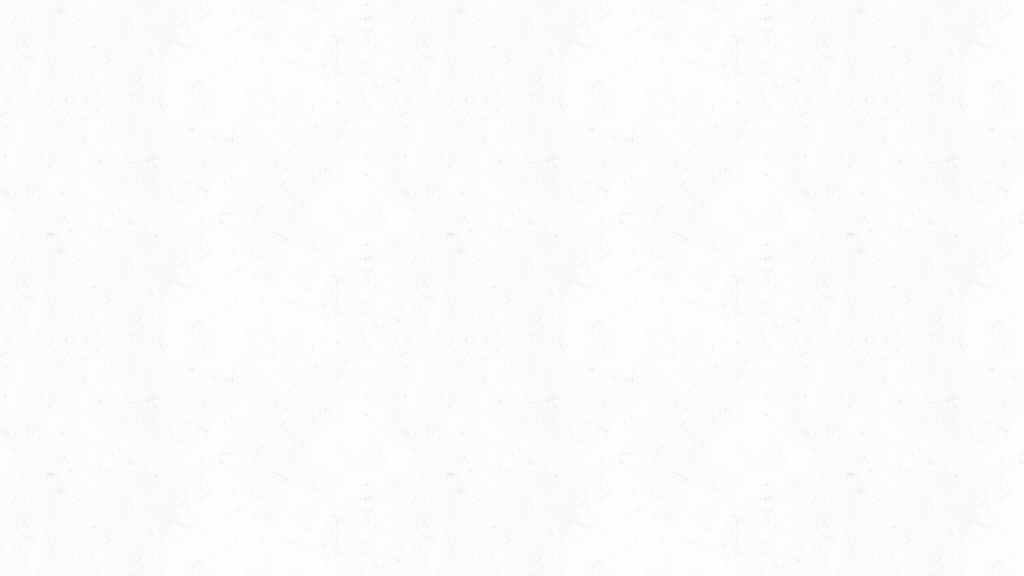
Mass defect
From the periodic table, we know the number of protons and neutrons in an atom of each element. For example, starting from top left:
hydrogen : 1 proton, 0 neutron
helium : 2 protons, 2 neutrons
lithium : 3 protons, 4 neutrons
and so on.
The datasheet in the exam lists the mass of a proton and a neutron. So we can easily use these values to calculate the masses of the above nuclei.
Right?
Wrong! If we just add the masses for 2 protons and 2 neutrons, the answer is a bit less than the actual mass of a helium nucleus. Likewise for all the other elements.
What is going on ?
The difference is called "mass defect". The reason is because when 2 protons and 2 neutrons come together from form a helium nucleus, some of the total mass is converted to energy. The amount of energy released is given by E = mc2, where m is the loss in mass - also called the "mass defect". This is Einstein's famous equation.
Strange?
In principle, this also happens when you drop a stone to the ground. The total mass of stone and Earth decreases a tiny tiny bit for the same reason - converted to heat on impact. So tiny that our weighing machine would not be able to measure it.
It also works the other way - if you pick the stone, total potential energy of stone and Earth in increases. So total mass increases according to m = E/c2, where E is the increase in potential energy of the stone.
Atomic Bomb
Little Boy is the name of the type of atomic bomb used in the bombing of the Japanese city of Hiroshima on 6 August 1945 during World War II.
In one of the most tragic demonstration of E = mc2, Little Boy was dropped from a plane over the Japanese city of Hiroshima on 6 August 1945.
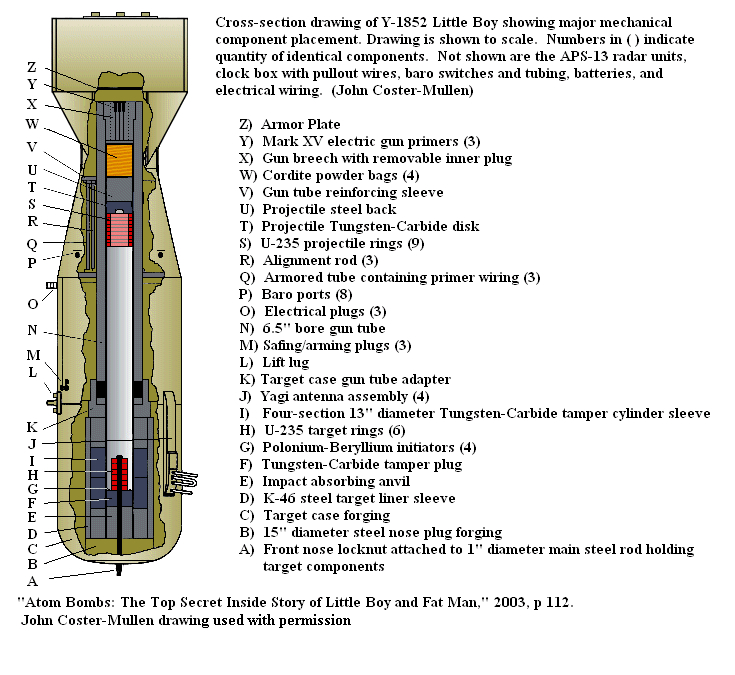
Little Boy has a total mass of 4,400 kg. It contain contained 64 kilograms of highly enriched uranium - meaning that the uranium has 20% or higher concentration of the uranium-235 isotope. "Isotope" refers to atoms with a particular number of neutrons.
The uranium was first separated into two parts in the shapes of a rod and a hollow cylinder at some distance a part. To detonate, the rod was simply pushed through the rings. This increased the concentration enough for the neutrons released to start a "chain reaction", giving a rapid conversion of mass to energy.
We may imagine that this energy starts off as very high kinetic energies of the product of the nuclear reaction:

From a human viewpoint, these would quickly turn into massive amount of heat - expanding and destroying everything in the way.
Binding Energy
The decrease in mass of Earth and stone when you drop the stone on the ground can be called a "mass defect", though this term is traditionally reserved for nuclear reactions.
Protons have positive charges and should repel each other. Neutrons are electrically neutral and do not exert electrical force on other particles. Imagine we have 2 protons and 2 neutrons. If they are somehow forced to come very close,a strong nuclear force takes over and they can actually join together.
2 protons and 2 neutrons joined together is actually the same as a helium nucleus. From the examples above, we would expect that some energy is given out, and the mass of the helium nucleus would be less that the total mass of the separate protons and neutrons.
This energy given out is called the "binding energy".
So, apart from hydrogen atom which has only 1 proton at the centre, the nucleus of an atom is made up of a number of protons and neutrons joined together. Somehow. Exactly "how" is not in this physics syllabus, but if you really like to know, you can google for phases like "strong nuclear forces" and "quarks".
For this article, I shall just focus on how the binding energy changes as the number of protons increases. As mentioned above, this corresponds to going from element to element along the periodic table. This is how the avearge binding energy per nucleon for each nucleus changes:

We can think of the binding energy as the energy needed to separate all the protons and neutrons in a nucleus completely, far away, out of reach from one another, ... In the nuclear scale, that probably means just more than one atom away.
This binding energy graph looks quite harmless. Actually, it has everything to do with the atomic bomb and nuclear energy today. When the uranium-235 (U235) nucleus split into krypton-92 and barium-141 nuclei, there is a decrease in total mass which is converted to energy.
The binding energy curve above tells us why. It is because the binding energy per nucleon for the fission products krypton-92 (92Kr) and barium-141 (141Ba) are higher.
Another way to say this: when protons and neutrons come together to form a krypton or barium nucleus, the average energy given out per nucleon is more than when they form a uranium-235 nucleus.
This explains why nuclear fission of uranium-235 can give out so much energy.
Can we apply the same idea to the left side of the graph? For example, can we take 4 hydrogen nuclei (1H) and merge them for form a helium nucleus ((4He)? The change in binding energy per nucleon is a lot bigger than uranium fission!
The answer is yes! The reaction is called nuclear fusion, since a number of protons are fused together for form the helium nucleus. And yes, the bomb is much more powerful than the uranium fission one.
Unfortunately, as of 2024, scientists have not yet succeeded in making a nuclear fusion power plant for peaceful use.
The reason is that "we need temperatures exceeding 100 million degrees Celsius and intense pressure to make deuterium and tritium fuse", according to the International Atomic Energy Agency. Guess it is a bit hard to make a container that can hold such temperature on Earth. Seems like peace is always harder than war.
Spontaneous and random nature of radioactive decay
We now turn to the topic of radioactive decay. This is a more gentle process than nuclear reaction, but still be a danger to health and safety if people are not careful with handling it.
We have seen an example of the destructive power of radioactive material when placed inside the intricately designed nuclear weapon. There are actually many less known uses of radioactive materials in our daily life.
For example, most household fire alarms contain a tiny bit of americium-241. It decays by emitting alpha particles and gamma ray. This ionises the air around a gap in the electric circuit in the alarm. When there is a fire, the smoke fills the gap and allows electric current to flow. This triggers the alarm.
Cobalt-60 gives out gamma ray that can be used to kill germs in food. Technetium-99 can be absorbed by body organs to locate damaged tissues. Tritium emits low energy radiation (beta) particles that cause a phosphor lining inside the tube to emit light - without batteries.
Useful?
Every element may have stable nuclei and radioactive nuclei. For the same element, the atoms must have the same number of protons - but number of neutrons can be different. For example, a hydrogen atom normally has a nucleus with just 1 proton.
But it is also possible for a hydrogen atom to have 1 proton and 1 neutron in the nucleus. This would be twice as heavy, but still have the same chemical reactions as the normal 1 proton version. So they are considered the same element. Though the version with additional neutron is also called deuterium.
Atoms with the same number of protons but different number of neutrons are called isotopes.
Here is a periodic table. Elements that have at least one stable version of nucleus are shown in blue.
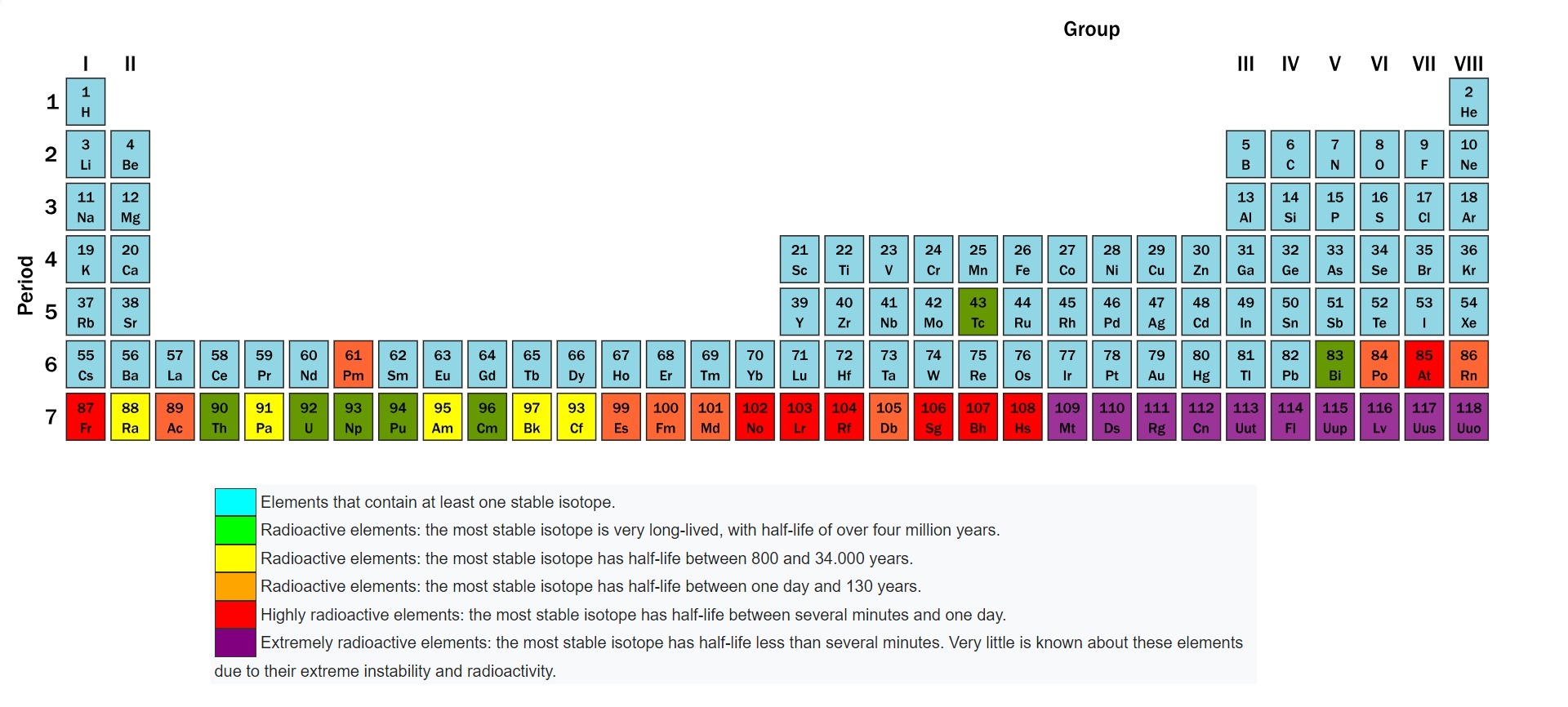
Elements shown in other colours are radioactive. Green ones have long half lives of millions of years. Purple ones have half lives less than a few minutes.
The graph below shows half lives of the elements. There is a lot of information packed into this graph. Z is atomic number (no. of protons), N is mass number (no. of protons and neutrons).
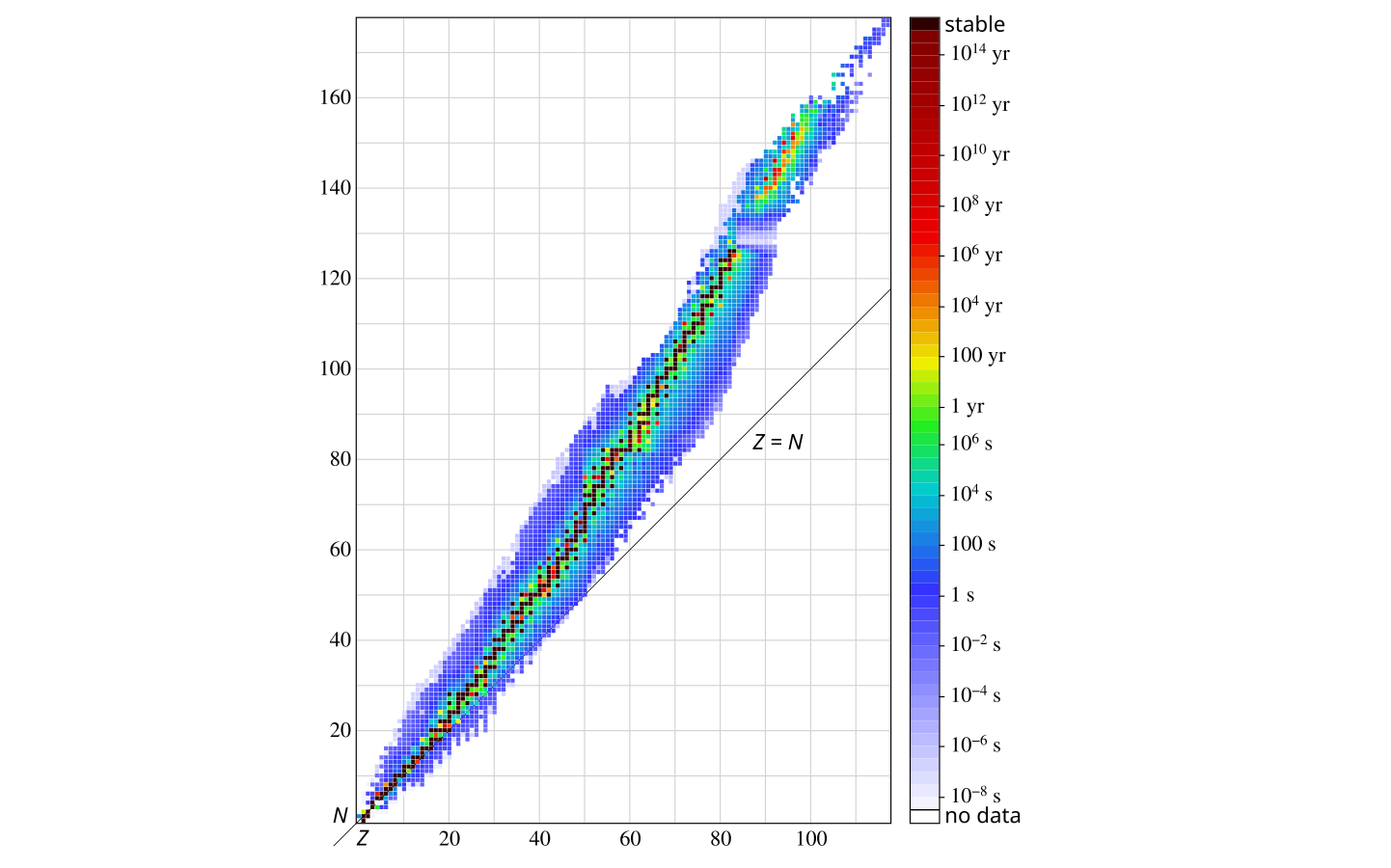
The horizontal axis shows the atomic number Z, so each number there means one particular element. E.g. Z = 1 is hydrogen, and Z = 92 is uranium.
The vertical axis on the left shows the mass number. This is the total number of protons and neutrons.
Notice that for every element - that is for each Z value - there can be a range of N values. This means each element must have atoms with the same number of protons, but can have atoms with different number of neutrons.
E.g. a carbon atom must have exactly 6 protons, but can have 2 to 16 neutrons! Have a look at the Wikipedia page if you are curious.
Carbon atoms in nature are mostly just the 6 neutron version. This version is the stable version, meaning it can exist forever. Errr - maybe. The 16 neutron one - has a half life of 6.2 ms So it would be mostly gone in 1 second, changed to other elements.
Background Radiation
Even if we do not have any radioactive materials in the room, it is still possible to measure some radiation. This is called background radiation. Where does this come from?
According to Wikipedia:
"Background radiation originates from a variety of sources, both natural and artificial. These include both cosmic radiation and environmental radioactivity from naturally occurring radioactive materials (such as radon and radium), as well as man-made medical X-rays, fallout from nuclear weapons testing and nuclear accidents."
So when people do measurements on radiation like α and β particles from radioactive materials, they must remember to measure the background radiation first. This must then be subtracted from the results when they measure the actual radioactive material.
Radiations
Radiation from decay of unstable nuclei are mainly of 3 types - α, β and γ.
α : particle with 2 protons, 2 neutrons
β : electron
γ : photon with higher energy than X ray photons
For the A level syllabus, there is one more particle that we need to know - the neutrino. With a mass of less than 2.14 × 10−37 kg, this is probably the lightest known particle - if we do not count the photon.
For comparison:
electron mass = 9.1093837 × 10-31 kg
proton mass = 1.67262192 × 10-27 kg
So the neutrino is more than 1 million times lighter than an electron. It also does not have electric charge. And rarely interact with other particles. For these reasons, it is very hard to detect.
So how was it discovered then? (Note: this in in the syllabus !)
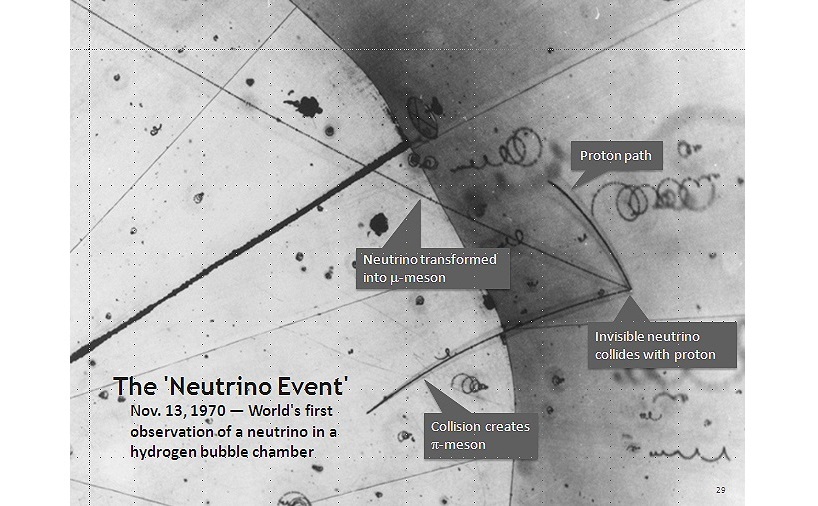
The experiment was carried out in what is called a "hydrogen bubble chamber". This contains liquid hydrogen, so we can imagine that it is really cold - very close to the hydrogen boiling point of −250.2 °C.
When a high speed subatomic particle zooms through the liquid, it would cause the liquid hydrogen along its path to start to boil. This has the effect of creating a trail of hydrogen bubble along the path, which can be quickly photographed.
From estimations of the curvature and kinetic energy of the particle's track, and knowing the magnetic field applied, it may be possible to estimate the mass of the particle. Note that in the beginning the hydrogen bubble chamber only has hydrogen, which consists of just protons and electrons.
The photo above shows 3 tracks of interest: a proton and 2 mesons. A meson is a particle that is a fraction of the mass of a proton. All 3 tracks show the particles moving to the left, so the proton must have have been hit by something invisible coming in from the right!
This is obvious from what we know of momentum conservation. Things can't just start moving to the left on their own. The photo is clear enough to confirm to the scientists that there is an invisible particle coming in from the right.
They named this particle the "neutrino". The symbol is the Greek letter ν, pronounced like the English word "new".
You can learn these concepts and more at Dr Hock's maths and physics tuition.